1) naturally occurring,
2) inorganic,
3) solids,
4) with a definite chemical composition, and,
5) an ordered internal structure.
What is a mineral? A mineral is a naturally-occurring inorganic (there are some exceptions to this) crystalline solid (though mercury is regarded as a mineral) with a specific chemical composition and a characteristic internal regular geometric arrangement of atoms, sometimes expressed as natural crystal faces.
A mineraloid is a substance that satisfies some, but not all of the parts of the definition. For example, opal, does not have a characteristic crystalline structure, so it is considered a mineraloid.
Glass - can be naturally formed (volcanic glass called obsidian), is a solid, its chemical composition, however, is not always the same, and it does not have a crystalline structure. Thus, glass is not a mineral.
Ice - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula H2O, and does have a definite crystalline structure when solid. Thus, ice is a mineral, but liquid water is not (since it is not solid).
Halite (salt) - is naturally formed, is solid, does have a definite chemical composition that can be expressed by the formula NaCl, and does have a definite crystalline structure. Thus halite is a mineral.
Minerals properties
Physical properties of minerals allow us to distinguish between minerals and thus identify them. Among the common properties used are:Habit - shape
Color Streak (color of fine powder of the mineral)
Luster (describe the light-reflecting characteristics of a mineral specimen. The luster of a specimen describes the general appearance of the specimen's surface in reflected light.)- metallic,
submetallic, nonmetallic (vitreous, dull, greasy, pearly, resinous, silky, waxy, and adamantine).
 Metallic Luster |  Submetallic Luster |
 Vitreous or Glassy (Nonmetallic) Luster |  Dull Luster |
 Greasy Luster |  Pearly Luster (nacreous luster) |
 Resinous Luster |  Silky Luster |
 Waxy Luster |  Adamantine Luster |
Cleavage (planes along which the mineral breaks easily)
Density (mass/volume)
Hardness (based on Mohs hardness scale).
Mohs Hardness Scale values are:
talc (1),
gypsum (2),
calcite (3),
fluorite (4),
apatite (5),
feldspar (6),
quartz (7),
topaz (8),
corundum (9),
Diamond (10).
Formation of Minerals
Minerals are formed in nature by a variety of processes. Among them are:Crystallization from melt (igneous rocks)
Precipitation from water (chemical sedimentary rocks, hydrothermal ore deposits)
Biological activity (biochemical sedimentary rocks)
Change to more stable state - (the processes of weathering, metamorphism, and diagenesis).
Precipitation from vapor. (not common, but sometimes does occur around volcanic vents)
Crystal Habits and Forms of Minerals
 Acicular crystals have a needle-like shape that tapers to a point or a blunt termination. Many acicular crystals can be clustered to produce fan-shaped or radially-shaped aggregates. The name acicular should be used when the length of an individual crystal is much greater than its width or diameter. Mineral examples include rutile, natrolite, millerite, and gypsum. |  Banded minerals have narrow layers or bands of different color and/or texture. These may be a response to changes in the composition of the growth liquid, the sedimentary process, or other conditions. Mineral examples: quartz (agate), malachite, rhodochrosite, and fluorite. The photo above shows rhodochrosite cabochons that display a banded habit. In one of the cabochons, the banded habit is actually an internal feature of a stalactitic habit. |
 Bladed crystals are elongated. They are much longer than they are wide, and their width exceeds their depth. They are shaped like a straight sword or knife blade. Their ends sometimes taper to a point. They might exist as single crystals, a cluster of many parallel crystals, or radiating clusters of crystals. Mineral examples: kyanite, actinolite, and stibnite. These blue crystals of kyanite have a bladed habit. Kyanite crystals are interesting because they have a hardness of 4.5 to 5 parallel to the length of their blades, and a hardness of 6.5 to 7 across the width of their blades. | 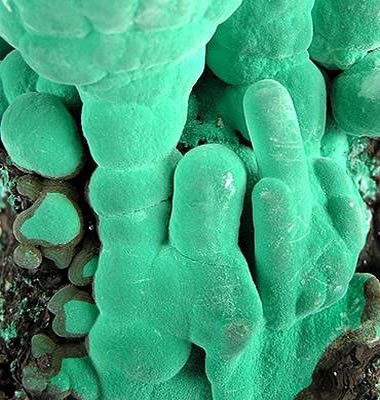 Botryoidal (also known as globular or mammillary) is derived from the Greek word botrys, which means "bunch of grapes". This habit name is used for crystal aggregates that have a globular or rounded shape. Mineral examples: hematite, malachite, smithsonite, hemimorphite, variscite, quartz (chalcedony), quartz (grape agate), and goethite. These green crystal aggregates of malachite have a botryoidal habit. |
 Columnar crystals are long prisms with enough width that the name acicular (or needle-like) does not apply. A single "column" might contain multiple parallel crystals. Mineral examples: calcite, tourmaline, and gypsum. These enormous crystals of selenite gypsum have a columnar habit. They are in the "Cave of the Crystals" cavern, Chihuahua, Mexico (a person in the lower-right quadrant of the photo serves as scale). These are some of the largest well-formed crystals in the world. |  Cubic crystals of pyrite. Fluorite and halite are two common minerals with a cubic shape. Cubes have six square faces and four-fold rotational symmetry around three axes. The photo shows cubic crystals of pyrite from Navajún, Rioja, Spain, that have grown in a marlstone. |
 Dendritic crystals form a branching pattern, much like the branches of a tree, the veins in a leaf, or the branching pattern of streams in a drainage basin. Mineral examples: copper, pyrolusite, and other manganese oxide minerals. These crystals of pyrolusite formed on a bedding surface of a piece of lithographic limestone collected near Solnhofen, Germany. |  Dodecahedral garnet crystals about four millimeters across, from Idaho. A dodecahedron is any polyhedron with twelve flat faces. The dodecahedron is one of the most common forms for garnet crystals. |
 Doubly terminated is a name used for a prismatic crystal that has a natural termination on both ends. Normally, crystals have a termination on one end - because the other end of the crystal was attached to the wall of a geode, the roof of a cavern, or a surface that it was growing on. The doubly terminated crystals shown in the photo above are composed of quartz and are known as "Herkimer diamonds" (a misnomer). |  Drusy is a habit name used for a surface that is covered with small crystals. The crystals themselves are referred to as a druse. Quartz is the most common mineral found as a druse. Other mineral examples: uvarovite garnet, malachite, and azurite. The rock in the photo above has a drusy surface because it is covered by a layer of uvarovite crystals. |
 Fibrous is a habit name used when minerals occur in very fine fiber-like crystals. They are often so fine that they look like fine hair. The habit also includes aggregates made up of a large number of parallel or radial fibers. Mineral examples: actinolite, chrysotile, serpentine, and tremolite. The actinolite crystals on this rock have a fibrous habit. Because of their fibrous shape (a roughly 1:20 aspect ratio) and properties, fibrous crystals of actinolite are regulated as asbestos. |  Foliated (also known as Micaceous) is a sheet-like or layered structure. Minerals with a foliated habit are often able to be split into thin sheets. Members of the mica family are the best examples of a foliated habit. Clay minerals and graphite can be described as having this habit, but their foliation is on a microscopic scale. Mineral examples: muscovite, biotite, and chlorite. This specimen of muscovite exhibits a foliated habit. The mineral can easily be separated into very thin sheets. |
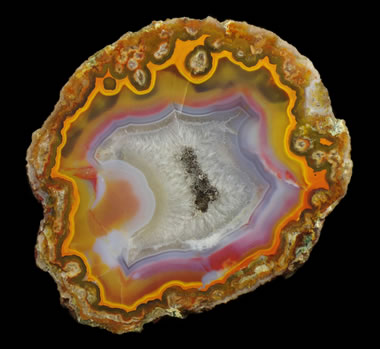 Geodic is a habit in which mineral aggregates form a rounded or oblate mass by crystallization on the inside walls of a cavity. Concentric bands or layers of mineral crystals subsequently develop, gradually infilling the cavity without infilling it completely and with a crystal-lined central void. The specimen in the photo is a geode formed by the precipitation of banded agate to form the external wall and initial layers. The center of the geode is lined with quartz crystals. |  Granular is the habit of a crystalline aggregate composed of many rounded or equant anhedral crystals of approximately the same size. The crystals might be loose with no interstitial material, or they might be interlocking such as calcite grains in a marble. Mineral examples: olivine, bornite, and scheelite. The photo shows a specimen of granular olivine in basalt. The olivine is in small rounded grains of about 2 to 4 millimeters in size with no interstitial material. |
 Hopper crystals are partially formed crystals that have experienced more rapid growth on their outer edges than in the center of the crystal. This causes them to be well-developed on the outer edges but less developed or "hollow" in the center. Halite (shown above) is one of the best-known examples of a mineral that sometimes displays the hopper crystal habit. Other mineral examples: galena and ice. |  Massive is the habit name used for masses of crystals that have no distinctive geometry. Most specimens of almost every mineral do not exhibit an obvious habit or obvious crystal form. Shown above is a specimen of lizardite serpentine from Wayne County, New York. The piece has no visible internal structure or characteristic external shape. |
 Nodular is the name of a habit in which mineral crystals grow to form rounded or bulbous structures. The crystals are usually arranged in a radial structure within the nodule, even though the nodules may exhibit concentric banding. In the concentric banding, each layer is composed of crystals growing up and outward from the layer immediately below. Mineral examples: quartz (agate), azurite, hematite, realgar, and variscite. A nodule of variscite (bright green), crandallite (canary yellow), wardite (gray) and montgomeryite (dark green). |  Octahedral diamond crystal, yellow in color and weighing 98.63 carats, recovered from the Jubilee (Yubileynaya) Pipe, Sakha Republic, Russia. The crystal measures approximately 29 x 28 x 27 millimeters and contains inclusions of olivine, graphite, and sulfide minerals. This diamond crystal is extremely interesting because its surface is covered with triangular dissolution features. Octahedrons have eight triangular faces and three axes of four-fold rotational symmetry. |
 Oolitic minerals occur in crystalline aggregates that are rounded and less than about four millimeters in size. Oolites form by chemical precipitation from a solution. Similar to pisolitic, but oolites are much smaller than pisolites. Mineral examples: hematite and calcite. |  Pisolitic minerals occur in crystalline aggregates that are rounded and about the size of peas. Individual pisolites are made up of many tiny radiating mineral crystals. They often develop a concentric structure formed when crystalline aggregate layers grow to enlarge the pisolites. Similar to oolitic, but pisolites are much larger than oolites. Mineral example: bauxite. |
 Prismatic is a habit name for minerals that form in elongated crystals with opposite faces normally parallel to one another. The crystals are often striated along their length (as in tourmaline) or across their width (as in quartz). Mineral examples: tourmaline, quartz, beryl, hornblende, augite, diopside, and topaz. Shown in the photo above are prismatic crystals of colorful tourmaline from Afghanistan with striations parallel to their long axis. |  Radiating crystal aggregates grow outwards from a central point. They consist of multiple crystals growing in diverging directions. Mineral examples: wavellite, pyrite, rutile, and kyanite. The photo above shows a massive aggregate of kyanite crystals, many of which form radial clusters. |
 Rosettes are clusters of tabular crystals in a radial arrangement that have an external geometry that resembles a rose or flower. Barite and gypsum sometimes form crystals of this shape in sand to produce a rosette with a sandy appearance. Mineral examples: barite, gypsum, pyrite, and marcasite. The photo shows a "barite rose" which formed when a cluster of barite crystals grew in sand, incorporating many of the sand grains within each crystal. |  Stalactitic is a habit name used for specimens that have formed as stalactites or stalagmites. The crystals often grow downwards or upwards in a cavity or cavern, yet they have a radial internal cross section. Mineral examples: calcite, malachite, goethite, and quartz. The photo shows a geode with stalactites of gem silica (inverted) from the Inspiration Mine, Gila County, Arizona. |
 Striations are fine, slightly indented lines that are present on the faces of some crystals. They always parallel a crystallographic axis and one of the edges of that crystal face. Mineral examples: pyrite, tourmaline, quartz, feldspar, euclase, and topaz. The photo shows a crystal of blue euclase with striations on its faces that parallel the long axis of the crystal. This specimen is also a good example of the prismatic crystal habit. |  Tabular crystals are flat and plate-like. They have lengths and widths that are much larger than their thickness. An easy way to describe their shape is to compare them to a tablet computer or a tablet that you use to write notes. Mineral examples: feldspar, topaz, barite, and corundum. The photo shows tabular segments of a corundum crystal that separated along planes of parting. |
References:
https://geology.com
https://www2.tulane.edu
http://www.galleries.com
https://australian.museum
