Physical Properties of Diamond
Chemical Classification Native element - CarbonColor Most diamonds are brown or yellow in color. The jewelry industry has favored colorless diamonds or those that have a color so subtle that it is difficult to notice. Diamonds in vivid hues of red, orange, green, blue, pink, purple, violet, and yellow are extremely rare and sell for high prices. A few white, gray and black diamonds are also cut and used as gems. Most industrial-grade diamonds are brown, yellow, gray, green and black crystals that lack the color and clarity to be a nice gem.
Streak Diamond is harder than a streak plate. Its streak is known as "none" or "colorless"
Luster Adamantine - the highest level of luster for a nonmetallic mineral.
Diaphaneity Transparent, translucent, opaque.
Cleavage Perfect octahedral cleavage in four directions.
Mohs Hardness 10. Diamond is the hardest-known mineral. However, the hardness of diamond is directional. It is hardest parallel to its octahedral planes and softest parallel to its cubic planes.
Specific Gravity 3.4 to 3.6
Diagnostic Properties Hardness, heat conductivity, crystal form, index of refraction, specific gravity and dispersion.
Chemical Composition C (elemental carbon)
Crystal System Isometric
Uses Gemstones, industrial abrasives, diamond windows, speaker domes, heat sinks, low-friction microbearings, wear-resistant parts, dies for wire manufacturing.
Diamond is a rare, naturally occurring mineral composed of carbon. Each carbon atom in a diamond is surrounded by four other carbon atoms and connected to them by strong covalent bonds - the strongest type of chemical bond. This simple, uniform, tightly-bonded arrangement yields one of the most durable and versatile substances known.
Diamond is the hardest known natural substance. It is also chemically resistant and has the highest thermal conductivity of any natural material. These properties make it suitable for use as a cutting tool and for other uses where durability is required. Diamond also has special optical properties such as a high index of refraction, high dispersion, and adamantine luster. These properties help make diamond the world's most popular gemstone and enable it to be used in specialty lenses where durability and performance are required.
 Diamond Crystal: A gem-quality diamond crystal in the rock in which it was formed. It is an octahedral crystal with triangular dissolution features on its surface and an estimated weight of about 1.5 carats. From the Udachnaya Mine, Yakutia, Siberia, Russia. |  Colored Diamonds: Diamonds can occur in a variety of beautiful colors. They include from top left, going clockwise: a heart-shaped diamond with a Fancy Vivid pink color weighing 0.70 carats; a Fancy Vivid yellowish-orange pear-shaped diamond weighing 0.85 carats; a Fancy Vivid yellow radiant cut diamond weighing 0.56 carats; a Fancy Deep brown radiant cut diamond weighing 1.00 carat; a Fancy Intense blue radiant cut diamond weighing 0.53 carats; and a Fancy Vivid green radiant cut diamond weighing 0.17 carats. They represent some of the finest hues of Fancy-color diamonds. |
Common Causes of Color in Diamond
Pink: Pink is a rare natural color in diamonds. It occurs when the diamond is subjected to stress within the Earth, and those forces cause glide planes of carbon atom displacement within the diamond crystal. When light passes through the planes, red light is selectively transmitted. The red light appears pink when the selective transmission is weak. The selective transmission is rarely strong enough to produce a red color.Red: Red diamonds are extremely rare in nature, and they are the most valuable diamonds when in a pure hue. Like pink diamonds, they have been subjected to stress which deformed the diamond crystal lattice, causing glide planes of carbon atom displacement. When light passes through the planes of displacement, the red wavelengths of light are selectively transmitted. Weak transmission of red light will produce a pink diamond.
Orange: Orange diamonds are very rare. The defect(s) that produce the orange color have not been determined with certainty and may vary from one orange diamond to another. The defects in orange diamonds cause them to selectively absorb blue light and selectively transmit orange.
Yellow: Yellow is the second most common natural color in diamonds. The color is usually caused by nitrogen atoms substituting for carbon in the diamond crystal lattice. This defect causes diamond to selectively absorb blue light and selectively transmit yellow.
Green: Green diamonds are very rare in nature. The color usually develops when high-energy radiation emitted by nearby radioactive mineral grains penetrates the diamond. The radiation knocks carbon atoms out of their position in the diamond crystal lattice, and that defect causes the diamond crystal to selectively absorb red light and selectively transmit green. Green color can also be a result of defects produced by the presence of nitrogen, hydrogen, or nickel within the diamond crystal.
Blue: Blue diamonds are rare in nature. The blue color is most often caused by boron atoms substituting for carbon atoms in the crystal lattice of diamonds that have formed at extreme depths. As little as one boron atom per million carbon atoms can produce a noticeable blue color. Boron in the diamond crystal causes the selective absorption of red light and the selective transmission of blue.
Violet: Violet is one of the rarest natural colors in diamond. It is sometimes caused by substitution of hydrogen in place of carbon in the diamond crystal lattice.
Purple: Purple is another rare color in diamond. In a study of 50 purple diamonds by GIA, they often found H3 and N3 color centers, sufficient enough to influence color. Purple diamonds and some pink diamonds modified by purple often exhibit color concentration along glide planes of carbon atom displacement.
Brown: Brown is the most common natural color in diamonds. The color develops when plastic deformation creates planes of missing and displaced carbon atoms in the diamond crystal lattice. These are known as glide planes, and they are where the brown color is concentrated. They can appear as a series of parallel color bands in the diamond known as "graining".
White: White diamonds occur in nature when the diamond has dense clouds of fine, reflective inclusions. The numerous inclusions can interfere with the passage of light and give the diamond a translucent or opalescent appearance.
Black: Black diamonds with a natural color usually contain such a high density of mineral inclusions that very little light passes through the gem. Common inclusions in black diamonds include graphite, pyrite, or hematite. Black color in heavily fractured diamonds can be caused by graphitization of the fracture surfaces.
Please note: The causes of color listed above are just a few of the many causes of color in natural diamonds. Numerous other natural defects can produce color. People also change or induce color in diamonds by treatments that include irradiation, heating, and coating - and by combinations of multiple treatments. There are many causes of color in diamonds, and researchers are only beginning to understand them.
Diamond cut
A diamond cut is a style or design guide used when shaping a diamond for polishing such as the brilliant cut. Cut does not refer to shape (pear, oval), but the symmetry, proportioning and polish of a diamond. The cut of a diamond greatly affects a diamond's brilliance; this means if it is cut poorly, it will be less luminous.Polish and symmetry are two important aspects of the cut.
A number of different diamond cuts have been developed. A diamond cut constitutes a more or less symmetrical arrangement of facets, which together modify the shape and appearance of a diamond crystal. Diamond cutters must consider several factors, such as the shape and size of the crystal, when choosing a cut.
 | 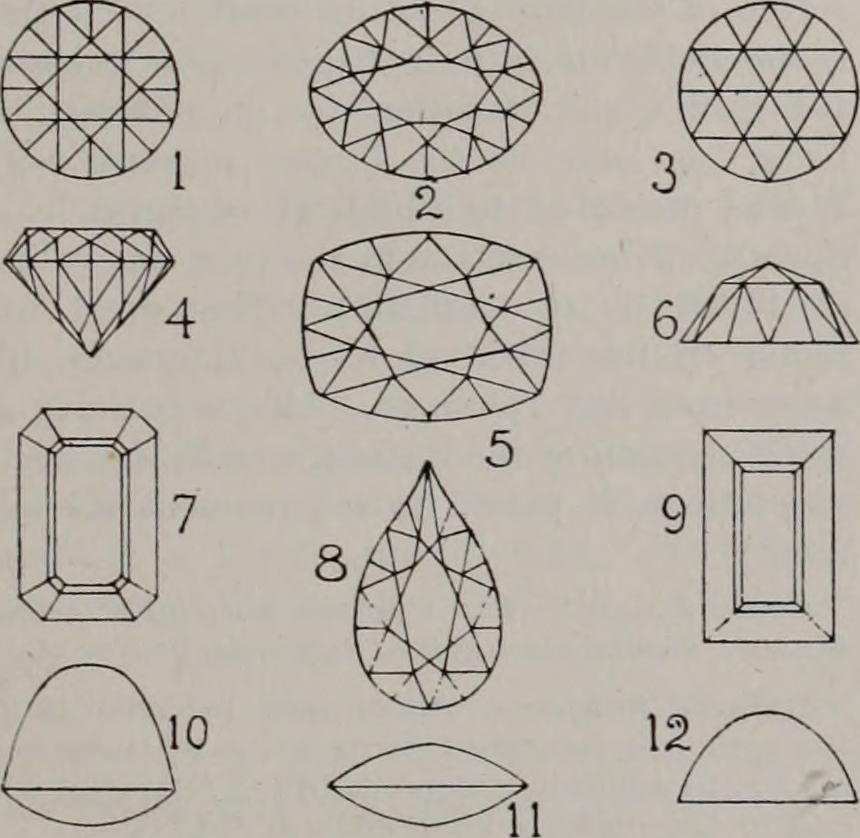 (1) Round brilliant, top view (2) Oval brilliant, top view (3) Rose cut, top view (4) Round brilliant, side view (5) Cushion brilliant, top view (6) Rose cut, side view (7) Step cut, octagon (8) Pear brilliant, top view (9) Step cut, oblong (10) High cabochon, side view (11) Cabochon, side view (12) Lentil-shaped, side view |
The choice of diamond cut is often decided by the original shape of the rough stone, location of internal flaws or inclusions, the preservation of carat weight, and popularity of certain shapes among consumers. The cutter must consider each of these variables before proceeding.
References:
https://geology.com
https://en.wikipedia.org
