What is a crystal? A crystal is an orderly arrangement of molecules formed because the component atoms and ions tend to aggregate together in certain specific patterns. Sometimes very different minerals are formed from the same starting materials (such as diamond versus graphite, both pure carbon) as determined by the temperature, pressure, or other conditions of formation.
Crystals structure is defined by the particular repeating arrangement of atoms throughout the crystal. The external appearance of the crystals is often related to its internal arrangement of atoms. There are six basic crystal systems:
| 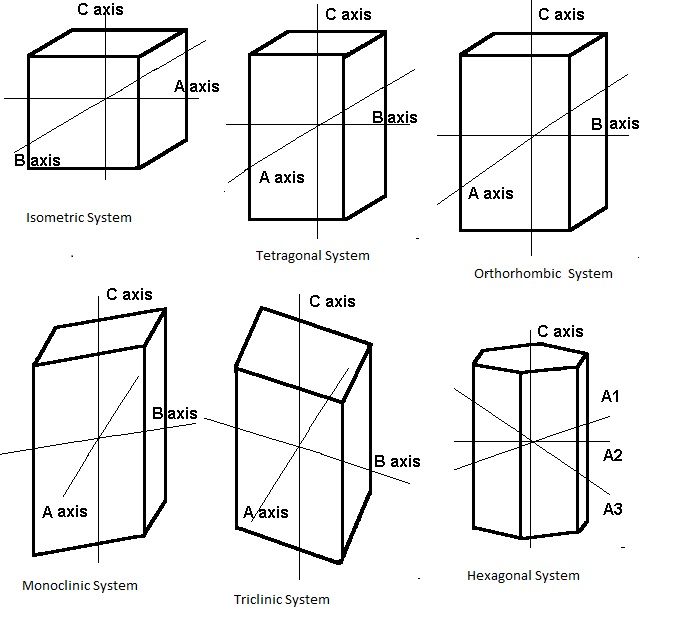 |
Each system is defined by a combination of three factors:
How many axes it has
The lengths of the axes
The angles at which the axes meet
Crystal structure depends on the conditions under which the mineral forms. Polymorphs are minerals with the same chemical composition but different crystal structures. The conditions are such things as temperature (T) and pressure (P), because these effect ionic radii.
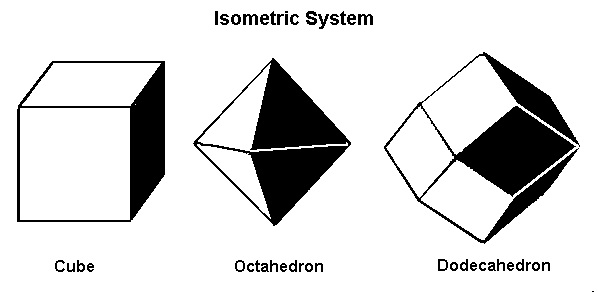
The Isometric System
The first and simplest crystal system is the isometric or cubic system. It has three axes, all of which are the same length. The three axes in the isometric system all intersect at 90º to each other. Because of the equality of the axes, minerals in the cubic system are singly refractive or isotropic.Minerals that form in the isometric system include all garnets, diamond, fluorite, gold, lapis lazuli, pyrite, silver, sodalite, sphalerite, and spinel.
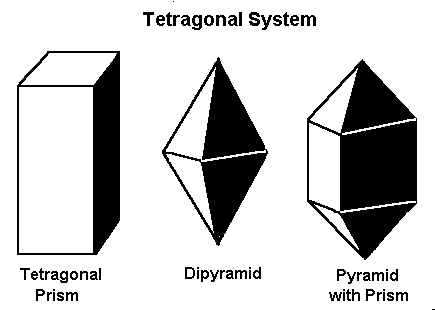
The Tetragonal System
The tetragonal system also has three axes that all meet at 90º. It differs from the isometric system in that one axis is longer than the other two axes, which are the same length.Minerals that form in the tetragonal system include apophyllite, idocrase, rutile, scapolite, wulfenite, and zircon.
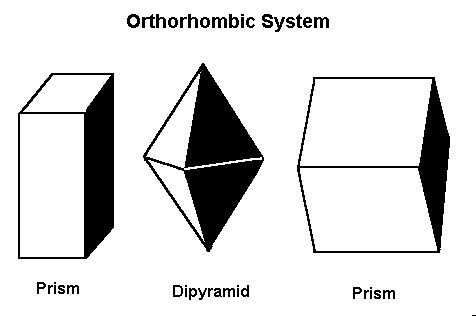
The Orthorhombic System
In this system there are three axes, all of which meet at 90º to each other. However, all the axes are different lengths.Minerals that form in the orthorhombic system include andalusite, celestite, chrysoberyl (including alexandrite), cordierite, iolite, danburite, zoisite, tanzanite, thulite, enstatite, hemimorphite, fibrolite/sillimanite, hypersthene, olivine, peridot, sulfur, and topaz.
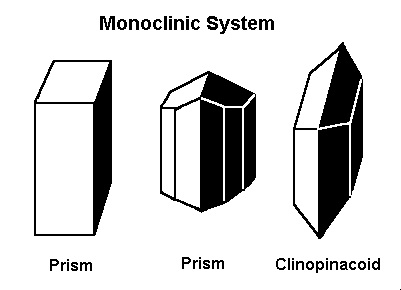
The Monoclinic System
The previously discussed crystal systems all have axes/sides that meet at 90º. In the monoclinic system, two of the axes, meet at 90º, but the third axis does not. All axes in the monoclinic system are different lengths.Minerals that form in the monoclinic system include azurite, brazilianite, crocoite, datolite, diopside, jadeite, lazulite, malachite, orthoclase feldspars (including albite moonstone), staurolite, sphene, and spodumene (including hiddenite and kunzite).
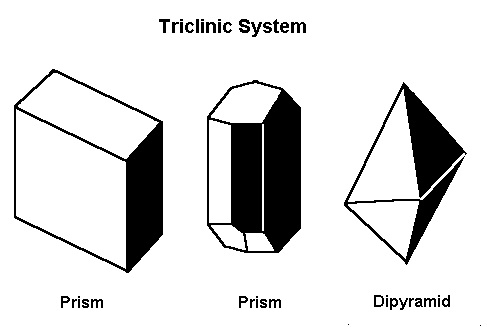
The Triclinic System
In the triclinic system, all the axes are different lengths. None of them meet at 90º.Minerals that form in the triclinic system include amblygonite, axinite, kyanite, microcline feldspar (including amazonite and aventurine), plagioclase feldspars (including labradorite), rhodonite, and turquoise.
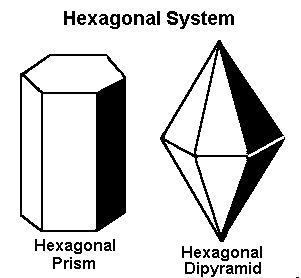
The Hexagonal System
The crystal systems previously discussed represent every variation of four-sided figures with three axes. In the hexagonal system, we have an additional axis, which gives the crystals six sides. Three of these are equal in length and meet at 60º to each other. The vertical axis is at 90º to the shorter axes.Minerals that form in the hexagonal system include apatite, beryl (including aquamarine, emerald, heliodor, and morganite), taaffeite, and zincite.
The Trigonal Subsystem
Mineralogists sometimes divide the hexagonal system into two crystal systems, the hexagonal and the trigonal, based on their external appearance. (Corundum, both ruby and sapphire, is sometimes described as trigonal). However, for gemological purposes, the above six categories are sufficient.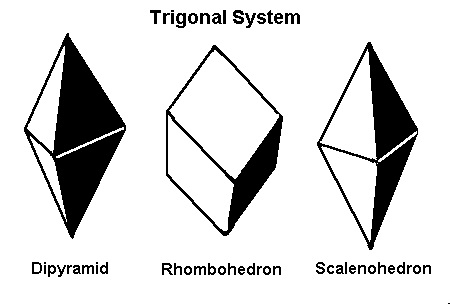

Gem Material Without Crystal Systems
Amorphous materials aren’t minerals because they don’t form in any of these crystal systems.Examples of amorphous materials used as gems include amber, glass (including obsidian), ivory, jet, moldavite, and opal.
Some materials used as gems may contain crystals of minerals but can’t themselves be described as crystals because they don’t have a uniform crystal structure. These materials are called polycrystalline.
Composition of Minerals
The variety of minerals we see depend on the chemical elements available to form them. In the Earth's crust the most abundant elements are as follows:O, Oxygen 45.2% by weight
Si, Silicon 27.2%
Al, Aluminum 8.0%
Fe, Iron 5.8%
Ca, Calcium 5.1%
Mg, Magnesium 2.8%
Na, Sodium 2.3%
K, Potassium 1.7%
Ti ,Titanium 0.9%
H, Hydrogen 0.14%
Mn, Manganese 0.1%
P, Phosphorous 0.1%
Note that Carbon (one of the most abundant elements in life) is not among the top 12.
The most common minerals are those based on Si and O: the Silicates. Silicates are based on SiO4 tetrahedron. 4 Oxygens covalently bonded to one silicon atom.
References:
https://uwaterloo.ca
https://www2.tulane.edu
http://www.galleries.com
https://www.gemsociety.org
