Atoms
Since minerals (in fact all matter) are made up of atoms, we must first review atoms. Atoms make up the chemical elements. Each chemical element has nearly identical atoms. An atom is composed of three different particles:Protons -- positively charged, reside in the center of the atom called the nucleus
Electrons -- negatively charged, orbit in a cloud around nucleus
Neutrons -- no charge, reside in the nucleus.
Each element has the same number of protons and the same number of electrons.
Number of protons = Number of electrons.
Number of protons = atomic number.
Number of protons + Number of neutrons = atomic weight.
Isotopes are atoms of the same element with differing numbers of neutrons. i.e. the number of neutrons may vary within atoms of the same element. Some isotopes are unstable which results in radioactivity.
Example: K (potassium) has 19 protons. Every atom of K has 19 protons. Atomic number of K = 19. Some atoms of K have 20 neutrons, others have 21, and others have 22. Thus atomic weight of K can be 39, 40, or 41. 40K is radioactive and decays to 40Ar and 40Ca.
Structure of Atoms
Electrons orbit around the nucleus in different shells, A Stable electronic configuration for an atom is one 8 electrons in outer shell Thus, atoms often loose electrons or gain electrons to obtain stable configuration. Noble gases have completely filled outer shells, so they are stable.Examples He, Ne, Ar, Kr, Xe, Rn.
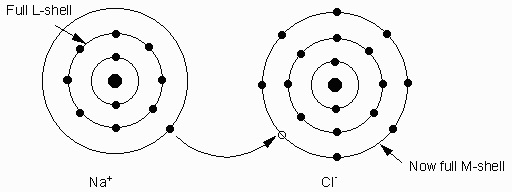
Others like Na, K loose an electron. This causes the charge balance to become unequal. and produce charged atoms called ions.
Positively charged atoms are called cations.
Elements like F, Cl, O gain electrons to become negatively charged. Negatively charged ions are called anions.
The drive to attain a stable electronic configuration in the outermost shell along with the fact that this sometimes produces oppositely charged ions, results in the binding of atoms together. When atoms become attached to one another, we say that they are bonded together.
Types of bonding:
Ionic Bonds - caused by the force of attraction between ions of opposite charge. Ionic bonds are moderately strong.Example: Na+1 and Cl-1. Bond to form NaCl (halite or salt).
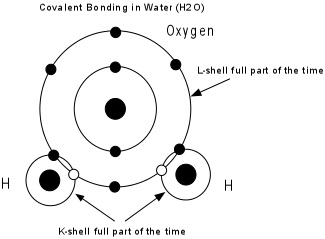
Covalent Bonds - Electrons are shared between two or more atoms so that each atom has a stable electronic configuration (completely filled outermost shell) part of the time. Covalent bonds are very strong bonds.
Example: H has one electron, needs to 2 to be stable. O has 6 electrons in its outer shell, needs 2 to be stable. So, 2 H atoms bond to 1 O to form H2O, with all atoms sharing electrons, and each atom having a stable electronic configuration part of the time.
Metallic Bonds - Similar to covalent bonding, except innermost electrons are also shared. In materials that bond this way, electrons move freely from atom to atom and are constantly being shared. Materials bonded with metallic bonds are excellent conductors of electricity because the electrons can move freely through the material.
Van der Waals Bonds - a weak type of bond that does not share or transfer electrons. Usually results in a zone along which the material breaks easily (cleavage). Examples: graphite and micas like biotite and muscovite.
References:
https://www2.tulane.edu
